CyTOF™
SINGLE-CELL PROTEOMICS
Every marker, all at once
CyTOF®
SINGLE CELL PROTEOMICS
Every marker, all at once
Phenotypic and functional variation can help cells adapt to dynamic conditions, but this heterogeneity challenges our ability to fully grasp different health and disease states. Understanding this complexity using rapid and unbiased high-dimensional proteomics with mass cytometry can generate actionable insights into molecular mechanisms and disease pathogenesis to refine targeted therapies and advance precision medicine approaches – ensuring nothing is missed.
Cytometry by time-of-flight or mass cytometry (on which CyTOF™ technology is based) is a cytometric technique that measures metal-conjugated antibodies bound to cell antigens. It is a pioneering technology developed to overcome many of the challenges experienced with fluorescence flow cytometry, increasing its utility by enabling the simultaneous assessment of a much higher number of parameters. This added capability maximizes the information obtained from each sample and, subsequently, each cell.
Download the CyTOF technology app note to learn more
How it works
Mass cytometry is an advancement in flow cytometry based on the principles of mass spectrometry with the goal of increasing the number of parameters on a cell that can be quantified simultaneously.
Cell targets are labeled with heavy metal-tagged antibodies and the atomic masses of the metals are measured. Ion counts are collected across each mass and combined to generate data with distinct signals not easily replicated in fluorescence-based approaches.
Diagram explanation
A mass cytometer contains inductively coupled argon plasma that breaks covalent bonds to produce free, charged atoms. The ion cloud is passed through a quadrupole to remove biological elements and isolate the mass tags, which are then separated by their mass-to-charge ratio in a time-of-flight mass spectrometer. Ion signals are collected per cell, resulting in single-cell measurements at subcellular resolution for downstream analysis. Data is compiled in a standard FCS file format that can then be input into a variety of standard software. Because mass cytometry leverages a single mass measurement for all parameters, this powerful system contains a single detector with a straightforward signal path from ionization to detection and the potential to detect up to 135 cell surface and intracellular antigens. This offers the quantitation and specificity of mass spectrometry in a flow cytometry-like approach.
Why mass cytometry
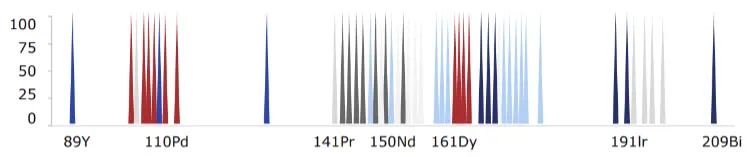
Mass cytometry is advantageous because of its ability to precisely and accurately distinguish between reporter molecules. By using atomic mass measurements, mass cytometry avoids autofluorescence signals from cells and tissues, enables fast panel design since all antibodies are used at once and facilitates barcoding to multiplex samples in large-scale acquisitions.
Mass spectrometry detects many different metal isotopes with high accuracy. By combining the single-cell analysis approach of flow cytometry with the precision of mass spectrometry, mass cytometry enables high-parameter analysis while generating little to no signal overlap. This eliminates the need to correct fluorescence spillover and further enables the use of statistical techniques to facilitate screening drug candidates and assessing on- or off-target effects post-treatment.
Download the CyTOF technology app note to learn more
Watch this detailed description of CyTOF technology
A mass cytometry workflow for quick and scalable panels
A mass cytometry workflow empowers the user to match metals to antibodies without having to manage color combinations or account for the proximity of molecules. This translates to the ability to quickly build and modify large panels with ease.

Or start with the validated, lyophilized, ready-to-go panel in a tube. Learn about the Maxpar™ Direct Immune Profiling Assay.
Simpler cytometry
With no autofluorescence issues and no signal overlap, many controls needed to manage fluorescence data are eliminated. Subsequently, compensation is not required for mass cytometry data, further simplifying the workflow and analysis and speeding up the time to actionable data.
How to incorporate intracellular markers
Two primary features of mass cytometry, metal stability and precision of measurement, allow for the simultaneous analysis of phenotype and function, enabling a detailed look into cells that is unmatched with other technologies. Due to their stability and robustness, metal tags are not susceptible to photobleaching or degradation from freezing and fixation. And the nature of mass spectrometry itself, in which cell material is cleared from the tags, offers comprehensive and accurate detection of all tags whether surface or intracellular, highly expressed or minimally expressed.
Because metal tags are very stable, samples can be fixed, frozen and stored for analysis at a later time or at another location. View this application note showing the high reproducibility of this workflow across multiple freezing time points of up to 120 days.
Learn about leveraging automation in mass cytometric analysis with the CyTOF XT system.
Applications of mass cytometry

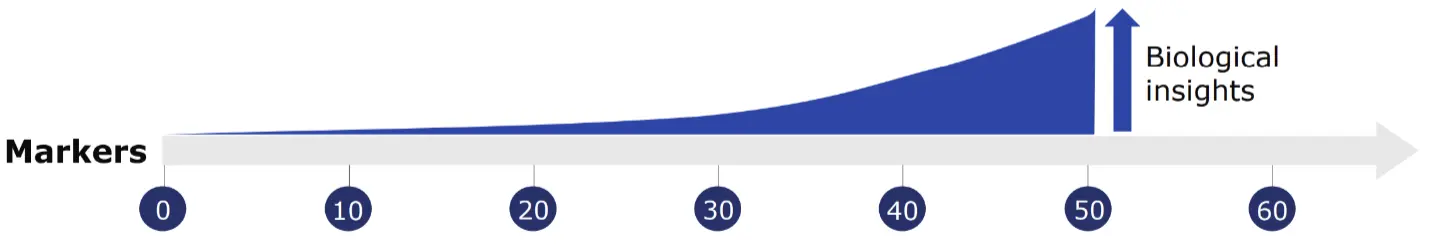
Global immune profiling details the immune response to disease or therapeutics, providing information on cell activation and function in diseased versus healthy states. For drug discovery, drug interaction with the immune system can be investigated to ensure efficacy and safety of a drug candidate. Immune profiling also offers insights into specific cell behaviors such as T cell exhaustion, B cell differentiation or NK cell activation. Mass cytometry is ideal for immune profiling, with the ability to measure over 50 parameters with high specificity and garner insights from raw data without the need for data manipulation through compensation or unmixing.
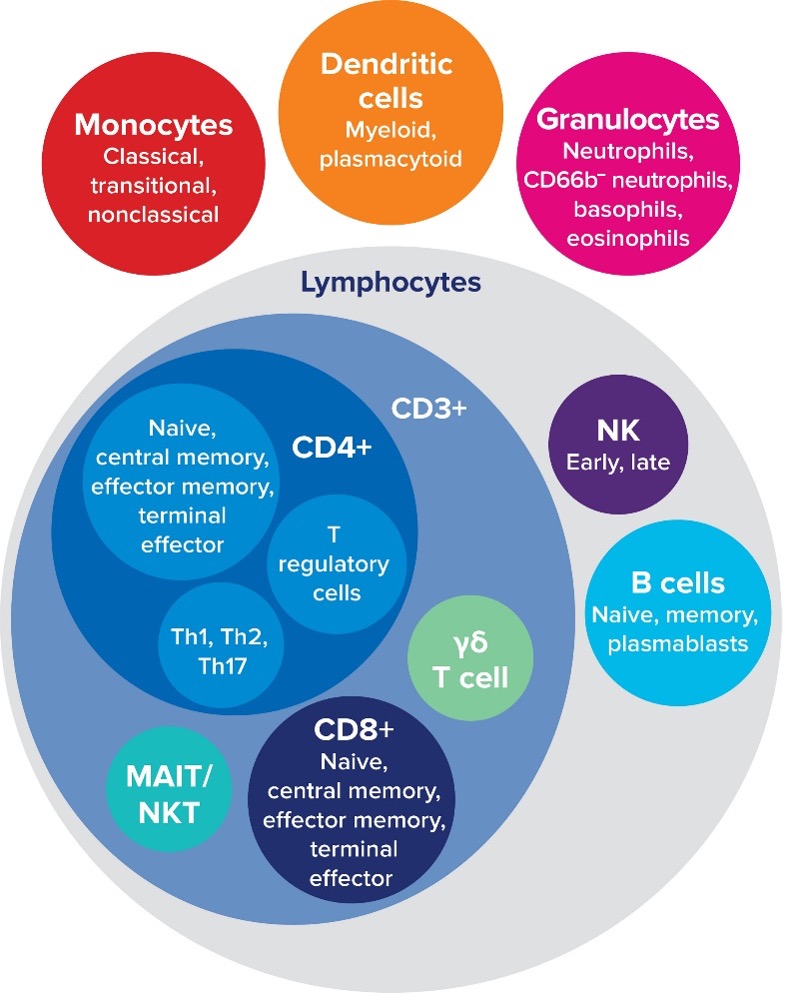
The Maxpar™ Direct Immune Profiling Assay makes immune profiling easier, designed as a simple single-tube workflow. It is a lyophilized assay that allows for the detection and characterization of major cell subsets in blood or bone marrow. The dry-format 30-marker antibody panel and automated Maxpar Pathsetter software enable quick identification of 37 immune cell populations.

The discrete nature of metal tags used in mass cytometry allows for the combination of functional and surface markers in a single tube, enabling phenotyping and comprehensive functional analysis. In fluorescence flow cytometry, the excitation light and emission signal cannot effectively pass through the layers of the cell structure. In contrast, mass cytometry atomizes the cell, breaking it into individual atoms, which allows for more straightforward and accurate detection of intracellular markers, as there are no physical barriers to interfere with signal detection.
Read this article, in which Zinaida Good, PhD, an Instructor at Stanford University, utilized the high-parameter benefits of mass cytometry to broaden the scope of their standard CAR T cell assay, looking at phenotype and function at once, in her research investigating why CAR T cell immunotherapies succeed or fail in patients. This helped her discover CAR T regulatory cells that she found to be significant to therapeutic outcome.

Mass cytometry performs exceptionally well with autofluorescent samples, including those with high myeloid content, epithelial cells, retinal cells, neurons or fibroblasts. Because antibodies are metal tagged, the data will not have any autofluorescence interference, ensuring clear and accurate results even with challenging samples.

The high-parameter capabilities inherent in mass cytometry facilitate investigations of diverse cellular networks as coordinated systems, analyzing multiple cellular phenotypes and behaviors in a single sample. Scalable throughput is essential for thorough characterization of this type of complexity, where vital rare cell populations might otherwise be missed. This utility extends to further investigating cellular metabolism, including protein levels, posttranslational modifications and proteolysis products that can all be quantified from a single experiment.
For more information, read this article: What does high-parameter cytometry really mean?

When designing a panel, the use of metal tags eliminates concerns of overlapping emission/excitation spectra, and there is no need to account for the proximity of molecules. Users do not have to worry about cross laser excitation, fluorescence spreading, bleaching or fluorescence resonance energy transfer (FRET). When validating a panel, due to negligible overlay, dozens of markers can be titrated together in a single tube using minimal sample.

Mass cytometry has been expanded to explore spatial biology with the development of Imaging Mass Cytometry™ technology in 2017. Imaging Mass Cytometry brings the high-parameter precision of mass cytometry to tissue imaging. All of the same advantages that apply to cell suspensions are also leveraged in staining tissues with metal-conjugated antibodies, generating clear images with little to no background issues or autofluorescence challenges.
A phospho CyTOF approach assesses signaling pathways using antibodies targeting phosphorylated proteins. Using this approach, researchers can detail cell populations based on surface receptor expression patterns in addition to dynamically characterizing immune activation. The application is well suited for measuring small changes above background, common with low-abundance phosphoproteins, and for deeper phenotyping and more comprehensive profiling of these phosphoproteins compared with fluorescence flow cytometry.
EpiTOF (epigenetic landscape profiling) is a powerful platform that allows highly multiplexed analysis of chromatin modifications in single cells and facilitates the identification of global changes of chromatin marks between biological samples. This may be broadly employed to better understand the roles of epigenetic mechanisms in the regulation of hematopoietic differentiation and immune cell physiological functions. Since EpiTOF is an analytical approach based on CyTOF technology, it enables the simultaneous measurement of epigenetic and immunological markers at the single-cell level.

Mass cytometry and fluorescence flow cytometry are often used together, the former for high-parameter measurements and discovery and the latter for smaller panel validations.
Mass cytometry and scRNA-seq can be combined for deeper phenotypic and functional characterization of diverse cell populations in a single sample. Mass cytometry can also be used to confirm scRNA-seq data. Applying a multimodal approach to analyze the complexity and heterogeneity of microenvironments such as those associated with tumors or inflammation generates meaningful data pertinent to determining therapeutic effects or identifying new targets as well as correlating immune signatures with treatment response.