Exploring neurodegenerative diseases
How multiplex imaging technologies expedite the discovery of valuable biological insights into neurodegenerative conditions such as Alzheimer’s disease
During Alzheimer’s & Brain Awareness Month, the Alzheimer’s Association and other focused organizations look to help end Alzheimer’s and all other dementia by accelerating research, driving risk reduction and early detection, and maximizing care and support. Dementia, an umbrella term for loss of memory and other thinking abilities, affected more than 55 million people worldwide in 2019; Alzheimer’s disease accounts for 60–80% of dementia cases, making deciphering and understanding this disease and its etiology a priority for many researchers.
Neurodegenerative diseases (NDs) are chronic progressive conditions of the central nervous system that occur when there is a loss of cellular structure or function. NDs affect millions and millions of people around the world – approximately 15% of the global population – resulting in disability and dependency as well as physical, psychological, social and economic impacts for people living with NDs, their carers and their families. And since the likelihood of developing a neurodegenerative condition rises dramatically with age, these numbers will grow every year as life expectancy increases.
No cures currently exist for NDs and there is no way to slow their progression, which makes neurodegeneration research so crucial and timely. However, this research is also extremely challenging: The brain is a complex organ with many intricate processes that, if they go awry, could lead to the deterioration of neuronal tissue. Brain tissue is also very heterogeneous and inherently autofluorescent, presenting technical challenges when using conventional fluorescence-based imaging techniques. Furthermore, NDs often vary from person to person, affecting people in different ways and with a variety of symptoms. Being able to fully address the brain’s complexity means clarifying the underlying molecular mechanisms driving neurodegeneration and using technology and tools that enable comprehensive assessment of the structural and cellular organization of the heterogeneous brain regions.
Transcriptomics – used for examining changes in transcriptional regulation and identifying differentially expressed genes – has traditionally been utilized for neuroscience imaging but ultimately can’t paint a complete picture of the changes occurring in the Alzheimer’s disease brain due to a lack of spatial information and because transcriptomic analysis doesn’t provide comprehensive insight into protein profiles.
Proteomics, which examines the interactions, function, composition and structures of proteins and their cellular activities, complements other omics studies by providing an essential bird’s-eye view into Alzheimer’s disease origin and development. Proteomics enables the detection of post-translational protein modifications – such as protein accumulation, aggregation, phosphorylation or oxidation – that are known to play key pathological roles in Alzheimer’s disease; and permits the discovery of novel proteins involved in Alzheimer’s pathogenesis, all while using only small amount of brain tissue.
Technologies that can provide detail and specificity of the brain’s inner workings are starting to help us understand the pathology of neurodegenerative conditions. A recent application note from Standard BioTools, Exploring Neurodegenerative Diseases With Imaging Mass Cytometry, details how Imaging Mass Cytometry™ (IMC™) is one of these technologies, capturing key biological insights into Alzheimer’s disease using a specialized panel for exploring different populations of neurons that contribute to aggregate formation.
IMC technology is a spatial biology tool that offers the ability to comprehensively visualize the pathological features of disease. Unlike traditional cyclic fluorescent methods, IMC platforms can uncover the spatial distribution of 40-plus distinct protein markers simultaneously without tissue degradation and autofluorescence artifacts. For Alzheimer’s disease, this can be incredibly useful for the characterization of mechanisms of action for disease pathogenesis or to understand initiators of early neurodegeneration.
For example, recent work used IMC technology to correlate cell senescence to Alzheimer’s progression and distinguish potential mechanisms responsible for initiating senescence pathways in the microglia that could be applied to the design of future therapies targeting disease-associated senescence. Multiplexed IMC technology revealed overlapping expression of senescence and cell type specific markers.
Another set of studies identified and analyzed factors that could initiate early neurodegeneration, using IMC platforms to identify neuronal subtypes associated with Alzheimer’s disease and characterize their morphology and activation. First, characteristics and local pathology involving pTau and β-amyloid (Aβ) associated with vulnerable neurons were investigated, aimed at developing cell type-specific neuroprotective therapies. Further study provided evidence for a functional association between intra-Aβ accumulation and selective neuronal loss arising from intrinsic autophagy-related defects triggering early neurodegeneration. A panel of 31 antibodies for IMC systems generated a single-cell multiplexed protein expression spatial dataset of excitatory and inhibitory neuronal subpopulations, glial cells and disease pathology.
Additional advances in IMC technology are pushing neurobiology to expand what can be visualized for an even more detailed look into health and disease.
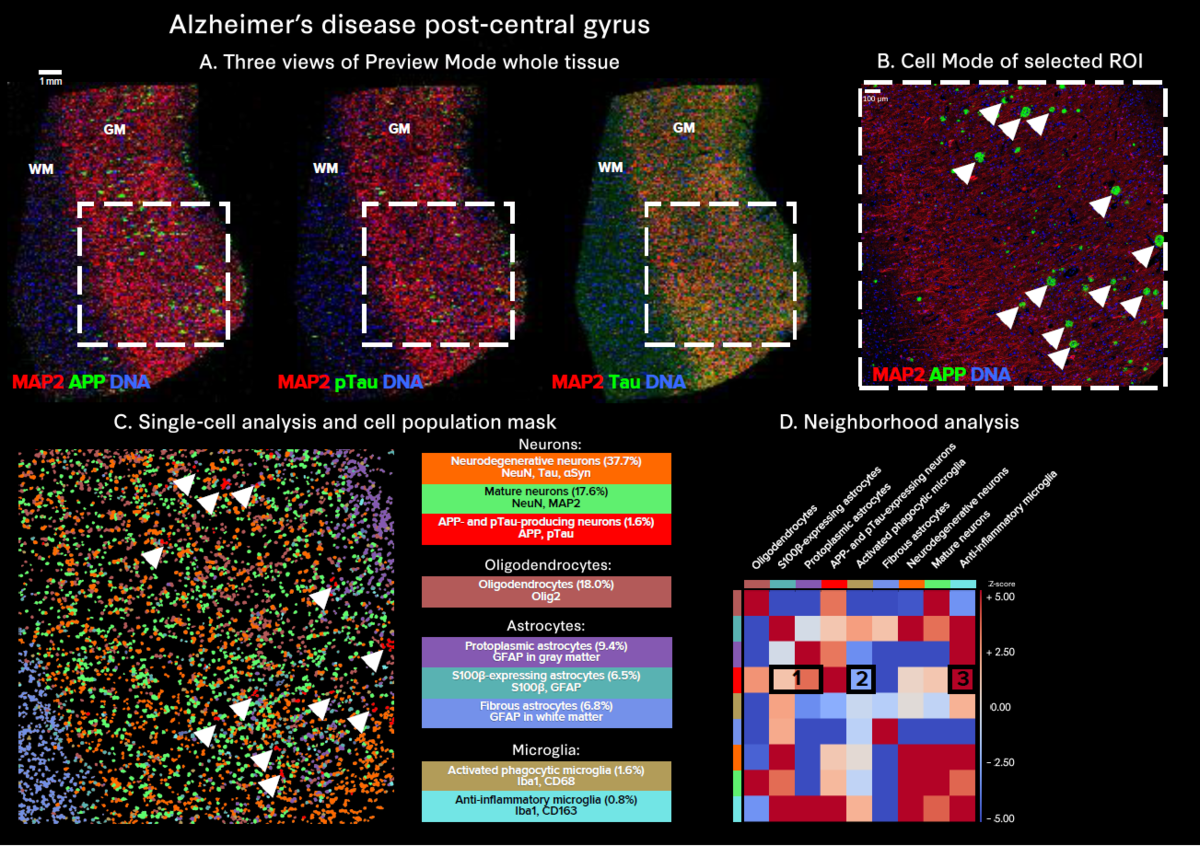
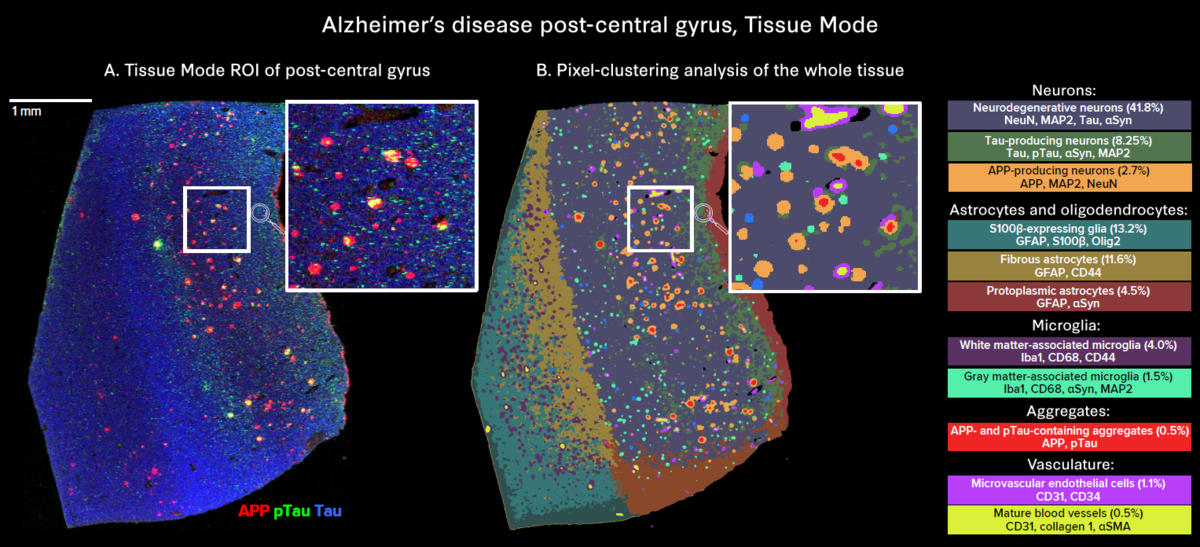
The use of whole slide imaging modes enables in-depth exploration of tissue heterogeneity:
- Preview Mode allows for rapid, simultaneous 40-plus-marker visualization across an entire tissue section, facilitating the identification of regions of interest for use in Cell Mode analysis
- Cell Mode and downstream single-cell analysis provide detailed insights at the highest resolution, uncovering distinct neuronal populations and their protein expression profiles, which is crucial for understanding the mechanisms of disease
- Tissue Mode enables comprehensive visualization of all markers, revealing the heterogeneity of aggregate distribution across the whole tissue
Three specialized panels are also available to support neurodegenerative spatial biology studies:
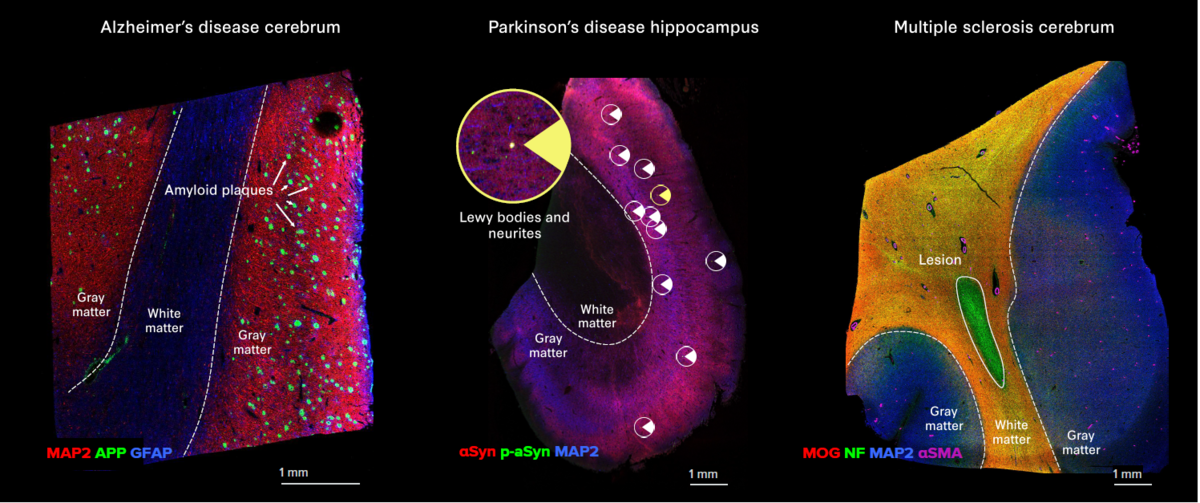
- The Alzheimer’s Disease IMC Panel, 3 Antibodies identifies amyloid precursor protein and Tau tangles
- The Parkinson’s Disease IMC Panel, 3 Antibodies identifies Lewy bodies and Lewy neurites
- The Multiple Sclerosis IMC Panel, 3 Antibodies identifies the loss of major myelin components
These products can easily be combined with other relevant IMC panels in the Standard BioTools™ catalog, allowing users to build tailored high-parameter panels with comprehensive coverage of key targets to better understand NDs.
Unless explicitly and expressly stated otherwise, all products are provided for Research Use Only, not for use in diagnostic procedures. Find more information here.
